
THE JANA LAB
2026
76. Kangkan Pradhan, Sourav Mandal, Kartic Manna, Ranjan Jana*



2025
77. Subhodeep Das, Sk Abdur Rahaman, Supriyo Das, Kangkan Pradhan, Ranjan Jana*


76. Kangkan Pradhan, Sourav Mandal, Ranjan Jana*
A Visible-Light Organophotoredox-Catalyzed anti-Markovnikov Hydrocarbamoylation of Unactivated Alkene and Cyclization Cascade via Radical Relay, 2025, Organic Letters




2024
73. Shuvam Mondal#, Shantanu Nandi#, Subhodeep Das, Ranjan Jana* A chemoselective hydroxycarbonylation and 13 C-labeling of aryl diazonium salts using formic acid as the C-1 source, 2024, Chem. Commun., doi.org/10.1039/D4CC04758C.


72. Subhodeep Das, Amit Banerjee, Pritha Das, Ranjan Jana∗ Visible-Light Organophotoredox Catalyzed Phosphonoalkylation of Alkenes via Deaminative Three-Component Radical–Radical Coupling, 2024, Synlett, doi.org/10.1055/a-2427-7689.
*on the occasion of 75th Birthday of Prof. B. C. Ranu.


71. Kasarla Varalaxmi, Kangkan Pradhan, Hasina Mamataj Begam, Arghya Polley, Deepak Kumar, Ranjan Jana* Transition-metal-free iterative two-fold reductive coupling and 1, 3-borotropic shift to form 1, 4-skipped dienes, 2024, Org. Biomol. Chem., doi.org/10.1039/D4OB01389A.


70. Subhodeep Das, Sk Abdur Rahaman, Kangkan Pradhan, Ranjan Jana∗ Organophotoredox-Catalyzed Synthesis of Unnatural α/β Amino Acids and Peptides via Deaminative Three-Component Coupling, 2024, Org. Lett., doi.org/10.1021/acs.orglett.4c02152


69. Ranjan Jana∗, Kangkan Pradhan Shining light on the nitro group: distinct reactivity and selectivity, 2024, Chem. Commun.,doi.org/10.1039/D4CC02582B


68. Shuvam Mondal, Ranjan Jana∗ Green light-mediated dual eosin Y/PdII-catalyzed C(sp2)–H arylation of N–H unprotected 2-arylquinazolinones, 2024, Org. Biomol. Chem, doi.org/10.1039/D4OB00779D


2023
67. Hasina Mamataj Begam, Kangkan Pradhan, Kasarla Varalaxmi, Ranjan Jana∗HFIP-assisted, cobalt-catalyzed three-component electrophilic C-H amination/cyclization/directing group removal cascade to naphtho[1,2-d]imidazoles, 2023, ChemComm, doi.org/10.1039/D3CC00749A


66. Shantanu Nandi, Pritha Das, Subhodeep Das, Shuvam Mondal, Ranjan Jana∗ Visible-light-mediated β-acylative divergent alkene difunctionalization with Katritzky salt/CO2, 2023, Green Chem.,doi.org/10.1039/D3GC00143A


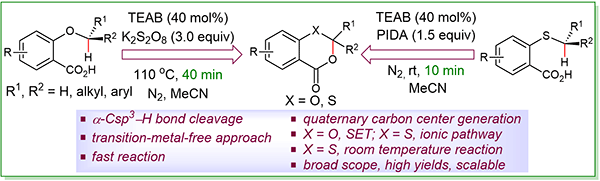
65. Kartic Manna, Hasina Mamataj Begam, Ranjan Jana∗ Transition-Metal-Free Dehydrogenative Cyclization via α-Csp3–H Activation of Ethers and Thioethers, 2023, Synthesis, DOI: 10.1055/a-2017-6065

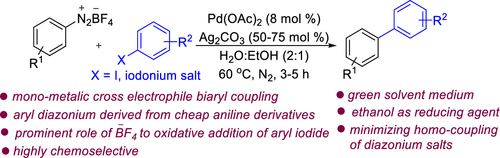
64. Manna, K.; Jana, R.* Palladium-Catalyzed Cross-Electrophile Coupling between Aryl Diazonium Salt and Aryl Iodide/Diaryliodonium Salt in H2O−EtOH, 2023, Org. Lett. , doi.org/10.1021/acs.orglett.2c03932

2022
63. Nandi, S.; Mondal, S.; Jana, R.* Protocol for chemo-and regioselective C (sp3)–H activation using a heterogeneous copper powderheterogeneous copper powdercatalyzed reaction, 2022, STARProtocols, doi.org/10.1016/j.xpro.2022.101781


62. Nandi, S.; Jana, R.* Toward Sustainable Photo‐/Electrocatalytic Carboxylation of Organic Substrates with CO2, 2022, Asian J. Org. Chem. doi.org/10.1002/ajoc.202200356


60. Begam, H. M.; Nandi, S.; Jana, R.* A Directing Group Switch in Copper-catalyzed Electrophilic C–H Amination/Migratory Annulation Cascade: Divergent Access to Benzimidazolone/Benzimidazole, Chem, Sci. 2022, 13, 5726-5733. doi.org/10.1039/D2SC01420C.
59. Das, P.; Das, S.; Jana, R.* Aryldiazonium Salts and DABSO: A Versatile Combination for Three-Component Sulfonylative Cross-Coupling Reactions, Chem. - Asian J. 2022, Ahead of Print. doi.org/10.1002/asia.202200
2021
58. Manna, K.; Ganguly, T.; Baitalik, S.*; Jana, R.* Visible-Light- and PPh3-Mediated Direct C–N Coupling of Nitroarenes and Boronic Acids at Ambient Temperature, Org. Lett. 2021,23, 8634–8639.ASAP,DOI: 10.1021/acs.orglett.1c03343.
57. Jana, R.*; Begam, H. M.; Dinda, E. The emergence of the C–H functionalization strategy in medicinal chemistry and drug discovery, Chem. Commun., Feature article, 2021, 57, 10842-10866 DOI: 10.1039/D1CC04083A.
56. Polley, A.; Varalaxmi, K.; Nandi, A.; Jana, R.* Divergent Total Synthesis of (±)-Mahanine and Other Carbazole Alkaloids, Asian, J. Org. Chem. 2021, 10, 1207-1215. DOI: 10.1002/ajoc.202100176
55. Das, P.; Das, S.; Varalaxmi, K.; Jana, R*. Metal-Free, Multicomponent Anti-Markovnikov Hydroarylsulfonylation and Alkoxyarylsulfonylation of Vinyl Arenes, Adv. Synth. Catal. 2021, 363, 575-584. DOI: 10.1002/adsc.202000995
2020
54. Dinda, E.; Bhunia, S. K.; Jana, R*. Palladium-Catalyzed Cascade Reactions for Annulative π-Extension of Indoles to Carbazoles through C-H Bond Activation, Curr. Org. Chem. 2020, 24, 2612-2633. DOI : 10.2174/1385272824999200817170058
53. Manna, K.; Begam, H. M.; Samanta, K.; Jana, R. Overcoming the Deallylation Problem: Palladium(II)-Catalyzed Chemo-, Regio-, and Stereoselective Allylic Oxidation of Aryl Allyl Ether, Amine, and Amino Acids, Org. Lett. 2020, 22, 7443-7449. DOI: 10.1021/acs.orglett.0c02465
2019
52. Das, P.; Begam, H. M.; Bhunia, S. K.; Jana, R.* Photoredox‐Catalyzed Tandem Demethylation of N,N‐Dimethyl Anilines Followed by Amidation with α‐Keto or Alkynyl Carboxylic Acids, Adv. Synth. Catal. Early view. DOI:10.1002/adsc.201900525
51. Bhunia, S. K.; Das, P.; Nandi, S.; Jana, R.*, Carboxylation of Aryl Triflates with CO2 Merging Palladium and Visible-Light-Photoredox Catalysts, Org. Lett. 2019, 21 (12), 4632-4637. DOI:10.1021/acs.orglett.9b01532
50. Hasina, M. B.; Choudhury, R.; Behera, A.; Jana, R.*, Copper-Catalyzed Electrophilic Ortho C(sp2)-H Amination of Aryl Amines: Dramatic Reactivity of Bicyclic System, Org. Lett. 2019, 21 (12), 4651-4656. DOI:10.1021/acs.orglett.9b01546
49. Bairy, G.; Nandi, A.; Manna, K.; Jana, R.*, Ruthenium(II)-Catalyzed Migratory C-H Allylation/Hydroamination Cascade for the Synthesis of Rutaecarpine Analogues, Synthesis, 2019, 51 (12), 2523-2531. DOI:10.1055/s-0037-1611525 (Invited special issue “Ruthenium in organic synthesis”)
2018
48. Bhunia, S. K.; Das, P.; Jana, R.*, Atom-economical selenation of electron-rich arenes and phosphonates with molecular oxygen at room temperature. Org. Biomol. Chem. 2018, 16 (47), 9243-9250. DOI:10.1039/c8ob02792g
47. Bairy, G.; Das, S.; Begam, H. M.; Jana, R.*, Exceedingly Fast, Direct Access to Dihydroisoquinolino[1,2-b]quinazolinones through a Ruthenium(II)-Catalyzed Redox-Neutral C-H Allylation/Hydroamination Cascade. Org. Lett. 2018, 20 (22), 7107-7112. DOI:10.1021/acs.orglett.8b03048
46. Polley, A.; Varalaxmi, K.; Jana, R.*, Palladium-Catalyzed Ortho C-H Arylation of Aniline Carbamates with Diazonium Salts under Mild Conditions: Expedient Synthesis of Carbazole Alkaloids. ACS Omega 2018, 3 (10), 14503-14516. DOI:10.1021/acsomega.8b02009
45. Polley, A.; Bairy, G.; Das, P.; Jana, R.*, Triple Mode of Alkylation with Ethyl Bromodifluoroacetate: N, or O-Difluoromethylation, N-Ethylation and S-(ethoxycarbonyl)difluoromethylation. Adv. Synth. Catal. 2018, 360 (21), 4161-4167. DOI:10.1002/adsc.201800824
44. Manna, M. K.; Bairy, G.; Jana, R.*, Sterically Controlled Ru(II)-Catalyzed Divergent Synthesis of 2-Methylindoles and Indolines through a C-H Allylation/Cyclization Cascade. J. Org. Chem. 2018, 83 (15), 8390-8400. DOI:10.1021/acs.joc.8b01034
43. Hossian, A.; Manna, K.; Das, P.; Jana, R.*, CuI/AgI-Promoted Decarboxylative Alkynylation of ortho-Nitrobenzoic Acids. Chemistry Select 2018, 3 (16), 4315-4318. DOI:10.1002/slct.201800758
42. Das, S.; Bairy, G.; Jana, R.*, Ligand-promoted γ-C(sp3)-H arylation and unsymmetrical diarylation to access unnatural amino acid derivatives. Org. Lett. 2018, 20 (9), 2667-2671. DOI:10.1021/acs.orglett.8b00874
2017
41. Singh, B. K.; Bairy, G.; Jana, R.*, A General Copper/Manganese Cocatalyzed C-H Selenation of Arenes, Heteroarenes, and Alkenes under Air, Chemistry Select, 2017, 2, 9227-9232. DOI:10.1002/slct.201701758
40. Hossian, A.; Manna, M. K.; Manna, K.; Jana, R.*, Palladium-catalyzed decarboxylative, decarbonylative and dehydrogenative C(sp2)-H acylation at room temperature, Org. Biomol. Chem. 2017, 15, 6592-6603. DOI:10.1039/C7OB01466J
39. Manna, M. K.; Bairy, G.; Jana, R.*, Dual visible-light photoredox and palladium(II) catalysis for dehydrogenative C2-acylation of indoles at room temperature, Org. Biomol. Chem. 2017, 15, 5899-5903. DOI:10.1039/C7OB01418J
38. Manna, M. K.; Bhunia, S. K.; Jana, R.*, Ruthenium(II)-catalyzed intermolecular synthesis of 2-arylindolines through C-H activation/oxidative cyclization cascade, Chem. Commun. 2017, 53, 6906-6909. DOI:10.1039/C7CC03053C
2016
37. Hossian, A.; Jana, R.*, Carboxyl radical-assisted 1,5-aryl migration through Smiles rearrangement, Org. Biomol. Chem. 2016,14, 9768-9779. DOI:10.1039/C6OB01758D
36. Singh, B. K.; Polley, A.; Jana, R.*, Copper(II)-Mediated Intermolecular C(sp2)−H Amination of Benzamides with Electron-Rich Anilines, J. Org. Chem. 2016, 81, 4295–4303. (one of the most read articles) DOI:10.1021/acs.joc.6b00659
35. Hossian, A.; Bhunia, S. K.; Jana, R.*, Substrate-Dependent Mechanistic Divergence in Decarboxylative Heck Reaction at Room Temperature, J. Org. Chem. 2016, 81, 2521-2533. DOI:10.1021/acs.joc.6b00100
34. Singh, B. K. S.; Jana, R.*, Ligand-Enabled, Copper-Promoted Regio- and Chemoselective Hydroxylation of Arenes, Aryl Halides, and Aryl Methyl Ethers, J. Org. Chem. 2016, 81, 831–841. DOI:10.1021/acs.joc.5b02302
2015
33. Bhunia, S. K.; Polley, A.; Natarajan, R.; Jana, R.*, Through-Space 1,4-Palladium Migration and 1,2-Aryl Shift: Direct Access to Dibenzo[a,c]carbazoles through a Triple C-H Functionalization Cascade- Chem. Eur. J. 2015, 21, 16786-16791. DOI:10.1002/chem.201503474
32. Chaudhary, T. Y.; Hossian, A.; Manna, M. K.; Jana, R.*, Chemo-, regio-, and stereoselective Heck–Matsuda arylation of allylic alcohols under mild conditions- Org. Biomol. Chem. 2015,13, 4841-4845. DOI:10.1039/C5OB00235D
31. Manna, M. K.; Hossian, A.; Jana, R.*, Merging C−H Activation and Alkene Difunctionalization at Room Temperature: A Palladium-Catalyzed Divergent Synthesis of Indoles and Indolines- Org. Lett. 2015, 17, 672-675. (highlighted in organic chemistry portal). DOI:10.1021/ol5036968
2014
30. Hossian, A.; Singha, S.; Jana, R.*, Palladium(0)-Catalyzed Intramolecular Decarboxylative Allylation of Ortho Nitrobenzoic Esters- Org. Lett. 2014, 16, 3934-3937. DOI:10.1021/ol5017349
29. Braverman, S.*; Cherkinsky, M.; Kalendar, Y.; Jana, R.; Sprecher, M.; Goldberg, I. Synthesis of water-soluble vinyl selenides and their high glutathione peroxidase (GPx)-like antioxidant activity- Synthesis, 2014, 46, 119-125.
28. Vaden, R. M.; Gligorich, K. M.; Jana, R.; Sigman, M. S.; Welm, B. E. The small molecule C-6 is selectively cytotoxic against breast cancer cells and its biological action is characterized by mitochondrial defects and endoplasmic reticulum stress- Breast Cancer Research, 2014, 16, 472.
27. Saini, V.; Liao. L.; Wang, Q.; Jana, R.; Sigman, M. S. Pd(0)-Catalyzed 1,1-Diarylation of Ethylene and Allylic Carbonates- Org. Lett. 2013, 15, 5008-5011.
26. Jana, R.; Pathak, T. P.; Jensen, K. H.; Sigman, M. S. Palladium(II)-Catalyzed Enantio- and Diastereoselective Synthesis of Pyrrolidine Derivatives, Org. Lett, 2012, 14, 4074-4077.
25. Jana, R.; Tunge, J. A., A Novel Recyclable Polymer Supported Rhodium(I) Catalyst for the C-C Bond Formation- J. Org. Chem. 2011, 76, 8376-8385.
24. Jana, R.; Partridge, J. J.; Tunge, J. A. Migratory Decarboxylative Coupling of Coumarins: Synthetic and Mechanistic Aspects- Angew. Chem. Int. Ed. 2011, 50, 5157-5161.
23. Liao, L.; Jana, R.; Urkalan, K. B.; Sigman, M. S. A Palladium-Catalyzed Three Component Cross-Coupling of Conjugated Dienes or Terminal Alkenes with Vinyl Triflates and Boronic Acids- J. Am. Chem. Soc. 2011, 133, 584-5787.
22. Jana, R.; Pathak, T. P.; Sigman, M. S., Advances in Transition Metal (Pd, Ni, Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners- Chem. Rev. 2011, 111, 1417-1492.
21. Fang, J. ; Jana, R.; Tunge, J. A.; Subramaniam, B., Continuous Homogeneous Hydroformylation with Bulky Rhodium-catalyst complexes retained by nano-filtration membranes- Applied Catalysis, A: General, 2011, 393, 294-301.
20. Braverman, S.; Cherkinsky, M.; Jana, R.; Kalendar, Y.; Sprecher, M. Reaction of Selenium and Tellurium Halides with Propargyl Alcohols. The Regio and Stereoselectivity of Addition to the Triple Bond- J. Phys. Org. Chem. 2010, 23, 1114-1120.
19. Chattopadhyay, K.; Jana, R.; Day, V.; Douglas, J.; Tunge, J. A. Mechanistic Origin of the Stereodivergence in Decarboxylative allylation- Org. Lett. 2010, 12, 3042-3045.
18. Pahadi, N. K.; Paley, M.; Jana, R.; Waetzig, S. R.; Tunge, J. A. Formation of N-alkylpyrroles via Intermolecular Redox Amination- J. Am. Chem. Soc. 2009, 131, 16626-16627.
17. Jana, R.; Trivedi, R.; Tunge, J. A., Mild Decarboxylative Allylation of Coumarins- Org. Lett. 11(15), 2009, 3434-3436.
16. Duan, S.; Jana, R.; Tunge, J. A., Lewis Acid Catalyzed Diastereoselective Hydroarylation of benzylidene Malonic Esters, J. Org. Chem. 2009, 74, 4612-4614.
15. Jana, R.; Tunge, J. A., A Homogeneous, Recyclable Rhodium(I) Catalyst for the Hydroarylation of Michael Acceptors- Org. Lett. 2009, 11, 971-974.
14. Samanta, S.; Adak, L. K.; Jana, R.; Mostafa, G.; Tuononen, H. M.; Ranu, B. C.; Goswami, S., Oxidative ortho-C-N Fusion of Aniline by OsO4. Isolation, Characterization of Oxo-Amido Osmium(VI) Complexes, and their Catalytic Activities for Oxidative C-C Bond Cleavage of Unsaturated Hydrocarbons- Inorg. Chem. 2008, 47(23), 11062-11070.
13. Ranu, B. C.; Chattopadhyay, K.; Ghosh, S.; Jana, R., Ionic-liquid promoted regio- and stereoselective addition of thiols to alkynes and alkenes- J. Indian Chem. Soc. 2008, 1199-1204.
12. Ranu, B. C.; Saha, A.; Jana, R., Microwave-assisted simple and efficient ligand-free copper nanoparticle catalyzed aryl-sulfur bond formation, Adv. Synth. Catal. 2007, 349, 2690-2696.
11. Braverman, S.; Jana. R.; Cherkinsky, M.; Gottlieb, H, E.; Sprecher, M. Regio- and stereospecific synthesis of functionalized divinyl selenides- Synlett, 2007, (17), 2663-2666.
10. Ranu, B. C.; Chattopadhyay, K.; Jana, R., Chemo-, Regio- and Stereoselective Addition of Triorganoindium reagents to acetates of Baylis- Hillman Adducts: A New Strategy for the synthesis of (E)- and (Z)- trisubstituted alkenes- Tetrahedron Lett. 2007, 48(22), 3847-3850.
9. Ranu, B. C.; Jana, R.; Sowmiah, S., An Improved Procedure for the Three-Component Synthesis of Highly Substituted Pyridines Using Ionic Liquid- J. Org. Chem. 2007, 72(8). 3152- 3154.
8. Ranu, B. C.; Banerjee, S.; Jana, R., Ionic Liquid as Catalyst and Reaction medium. The Remarkable Effect of a Basic Ionic Liquid, [bmIm]OH on Michael Addition and Alkylation of Active Methylene Compounds- Tetrahedron, 2006, 63(3), 776–782.
7. Ranu, B. C.; Chattopadhyay, K.; Jana, R., Ionic liquid promoted selective debromination of α-bromoketones under microwave irradiation- Tetrahedron 2006, 63(1), 155 - 159.
6. Ranu, B. C.; Jana. R., Ionic Liquid as Catalyst and Reaction Medium – A Simple, Efficient and Green Procedure for Knoevenagel Condensation of Aliphatic and Aromatic Carbonyl Compounds Using a Task-specific Basic Ionic Liquid, Eur. J. Org. Chem. 2006, (16), 3767-3770.
5. Ranu, B. C.; Jana, R., Ionic Liquid as Catalyst and Reaction Medium: A Simple, Convenient and Green Procedure for the Synthesis of Thioethers, Thioesters and Dithianes using an Inexpensive Ionic Liquid, [pmIm]Br, Adv. Synth. Catal. 2005, 347(14), 1811-1818.
4. Ranu, B. C.; Jana, R., Catalysis by Ionic Liquid. A Green Protocol for the Stereoselective Debromination of vicinal- Dibromides by [pmIm]BF4 under Microwave Irradiation- J. Org. Chem. 2005, 70(21), 8621-8624.
3. Ranu, B. C.; Jana, R., Direct Halogenation of Alcohols and Their Derivatives with tert-Butyl Halides in the Ionic Liquid [pmIm]Br under Sonication Condition – A novel, Efficient and Green Methodology, Eur. J. Org. Chem. 2005, 4,755-758.
2. Ranu, B. C.; Jana, R.; Samanta, S., A Simple, Efficient and General Procedure for Acetalization of Carbonyl Compounds and Deprotection of Acetals under the Catalysis of Indium(III) Chloride, Adv. Synth. Catal. 2004, 346(4), 446-450.
1. Ranu, B. C.; Jana, R.; Dey, S. S., An Efficient and Green Synthesis of 2-Arylbenzothiazoles in an Ionic Liquid, [pmIm]Br under Microwave Irradiation- Chem. Lett. 2004, 33(3), 274-275.
PATENTS
1. Polymer-supported Transition Metal Catalyst Complexes and Methods of Use- Tunge, J. A.; Subramaniam, B.; Fang, J.; Jana, R. U.S. Patent, No WO 2010057099.
Products
-
Developed a catalyst called JanaPhos following my name for biodiesel production from agricultural feedstocks.

































































